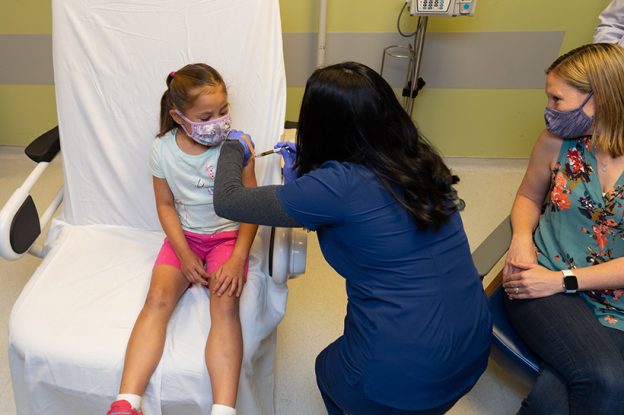
Kaiser Permanente Los Angeles Medical Center joins COVID-19 vaccine trial for children
Kaiser Permanente Los Angeles Medical Center is participating in a clinical trial for the Moderna COVID-19 vaccine in children ages 6 months to less than 12 years.
The KidCOVE study will evaluate the safety and efficacy of the Moderna COVID-19 vaccine (mRNA-1273), which is the same vaccine that was given Emergency Use Authorization (EUA) in December 2020 by the U.S. Food and Drug Administration for use in adults 18 years old and older.
“We know the vaccine is safe and prevents COVID-19 infection in adults, both from prior clinical trials and after being used in millions of patients under the EUA. The next important step is to study it in children.” said William Towner, MD, site principal investigator for the study and physician director of clinical trials for Kaiser Permanente Southern California.
This is the second COVID-19 vaccine trial that Kaiser Permanente Los Angeles Medical Center has participated in. Dr. Towner was the site principal investigator for the Pfizer-BioNTech trial at conducted there last summer.
“Although children seem to be at less risk from COVID-19 than adults, expanding the availability of safe and effective vaccines to younger populations is vital,” said Jerry Cheng, MD, chief of pediatrics at the Los Angeles Medical Center, and a co-investigator on the study.
“I’ve had many conversations with parents who already vaccinated themselves and are eager for the day when their kids will also have the protection of a COVID-19 vaccine,” said Dr. Cheng. “When we are able to offer safe and effective vaccines to children, it has the potential to benefit all of us by reducing transmission of the disease.”
Trial seeks to find appropriate doses, assess efficacy
The pediatric trial will include 2 different parts. The first part, underway now, is focusing on finding the appropriate dose for children of different ages. The second will focus on the vaccine’s effectiveness in preventing children from getting sick with COVID-19.
Enrollment will be staggered by age group, starting with children from 6 to 12 years old before moving to children between the ages of 2 to 6 years and finally to children under 2 years old.
All children participating in the first part of the study—the dosing phase—will receive 2 doses of the study vaccine about 28 days apart.
Children participating in the second part—the efficacy phase—will receive 2 doses of either the study vaccine or the placebo.
Moderna plans to enroll about 7,000 children in the trial. General information can be found on the KidCOVE study website. For more information about KidCOVE Study participation at the Los Angeles Medical Center location, Kaiser Permanente members can visit the local website here.
Kaiser Permanente is committed to researching a safe and effective vaccine that protects people of all ages and from all backgrounds against COVID-19. This includes ensuring clinical trial enrollment of a population that reflects the diversity of the community we serve.
Both Dr. Towner and Dr. Cheng are faculty members at the Kaiser Permanente Bernard J. Tyson School of Medicine.
Image: Avery Shih, 6, received her second COVID-19 vaccine dose on , as her mother, Erin Shih, watched.





